Background: Aberrant overexpression of pro-survival Bcl-2 family proteins is one mechanism by which leukemic cells circumvent apoptosis. BCL-2 overexpression is common in various subtypes of B-acute lymphocytic leukemia (ALL), including Philadelphia (Ph)-like ALL. Ph-like ALL is a chemotherapy-resistant disease associated with higher rates of induction failure and persistent measurable residual disease (MRD), and poor overall survival (OS) outcomes with frontline chemotherapy regimens, compared to other subtypes. Venetoclax (VEN) is a selective BCL2 inhibitor, and its addition to pediatric-type frontline regimens could improve response and long-term outcomes for B-ALL, including Ph-like subtype.
Design: This is a phase 1 study with an expansion cohort investigating the safety of combining VEN with induction (IND) and consolidation (CON) cycles of the CALGB 10403 backbone regimen (NCT05157971). Post CONS therapy was per the treating physician discretion. The study enrolled newly diagnosed adults [18-54 years (yrs)] with Ph-negative B-ALL. To enrich the study for Ph-like ALL, we excluded patients (pts) with KMT2A-rearranged, TCF3::PBX1, and ETV6::RUNX1 subtypes at diagnosis. Identification of Ph-like ALL was performed using RNA-sequencing, conventional cytogenetics, fluorescence in situ hybridization, and whole genome array studies. Dose level (DL) 1 of VEN was 400 mg orally daily on days 1-14 of IND and CON, with a ramp-up dosing during IND [Day 1= 100 mg, Day 2= 200 mg, Days 3-14= 400 mg/day].
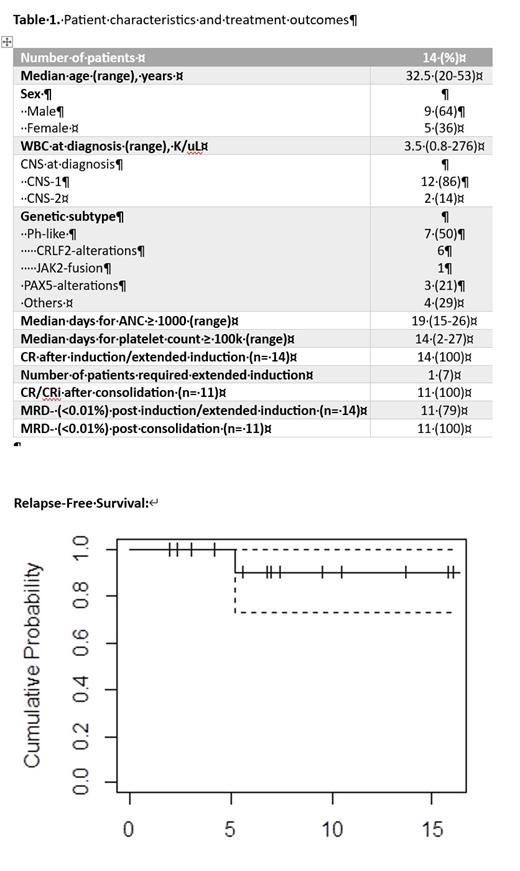
Results: We enrolled 14 pts as of July 1 st, 2023. The median age was 33 (20-53) yrs, of which 9 pts were males and 13 (93%) were Hispanics. Seven (50%) pts had Ph-like ALL (6 with CRLF2-alterations, 1 JAK2-fusion). Six pts were treated on the phase 1 part, and no dose limiting toxicity was observed during IND. Among 14 pts that completed IND, all achieved complete remission (CR), including 1 pt who required an extended IND phase, and 79% (n= 11) achieved MRD- (<0.01%) by multicolor flow (MCF) on day 28 of IND. Eleven pts completed CONS, and all achieved undetectable MRD CR or CR with incomplete count recovery (CRi) by MCF. Post CONS next-generation sequencing of MRD (clonoSEQ) was available for all 11 pts who completed CONS, and 7 (64%) were undetectable while 4 were detected at very low levels (range: 0.0007-0.0024%). Median time to neutrophil count ≥1000 and platelet count ≥100,000 were 19 (15-26) and 14 (2-27) days from starting IND, respectively. No pt died during IND or CONS cycles. Grade ≥3 adverse events at least possibly related to venetoclax and/or the C10403 regimen that occurred in ≥ 20% of evaluable pts during either IND or CONS cycles were leukopenia (100%), neutropenia (100%), lymphopenia (100%), anemia (70%), thrombocytopenia (50%), hyperbilirubinemia (30%) and hypertriglyceridemia (20%). Only 1 pt underwent consolidation with allogeneic stem cell transplant while in remission. With a median follow up for the entire cohort of 7.2 months (range: 1.9-16.1 months), only 1 pt with Ph-like ALL relapsed and he is currently alive.
Conclusion: The addition of 14 days of VEN at 400 mg daily dose to a pediatric-inspired regimen during IND and CONS was safe in adults with newly diagnosed B-ALL and did not delay counts recovery. The addition of VEN led to a promising higher than expected MRD- CR rate in high-risk B-ALL, including for pts with Ph-like subtype. The study is actively enrolling on the expansion cohort, and BH3 profiling on pre-treatment and post relapse samples will be performed to determine BCL-2 dependency and correlates to early MRD- response.
Disclosures
Aldoss:KiTE: Consultancy; Sobi: Consultancy; Jazz: Consultancy; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; Takeda: Consultancy. Aribi:Seagen: Consultancy; Kite, a Gilead Company: Consultancy. Koller:takeda: Consultancy, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; treadwell therapuetics: Consultancy, Other: safety review committee. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Ali:Karyopharm: Consultancy; GSK: Consultancy; Pharmaessentia: Consultancy; Blueprints: Speakers Bureau; BMS: Speakers Bureau; Incyte: Research Funding. Artz:Magenta Therapeutics: Other: Advisory Board; Astra Zeneca: Other: Advisory Board; Radiology Partner: Current equity holder in private company, Other: Spouse equity interest; Abbvie: Consultancy. Al Malki:Tscan: Consultancy. Salhotra:Jazz Pharma: Research Funding; Sanofi: Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees; Rigel Pharma: Research Funding; OrcaBio: Research Funding; BMS: Research Funding; Gilead: Research Funding; Kura Oncology: Research Funding. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Stein:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Pullarkat:Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal